In multicellular organisms, specific stem cell types with distinct developmental potentials occur during development. Transient pluripotent stem cells, which can differentiate into derivatives of all three germ layers (endoderm, ectoderm and mesoderm), are generated during blastocyst development. Adult stem cells, developing at later stages, are more restricted in their potential, since they can differentiate into progenitors and mature effector cell types of only one stem cell system. Adult stem cells have been identified in a variety of tissues in the adult organism and are important for lifelong tissue homeostasis and repair.
Stem cells are functionally defined by two unique attributes: their high self-renewal capacity and their multilineage differentiation potential. The presence of both characteristics in one cell is rare and sets these highly specialized cells aside from the majority of the somatic cell populations. These unique properties make stem cells ideal targets for stem cell engineering to generate cells with wanted properties.
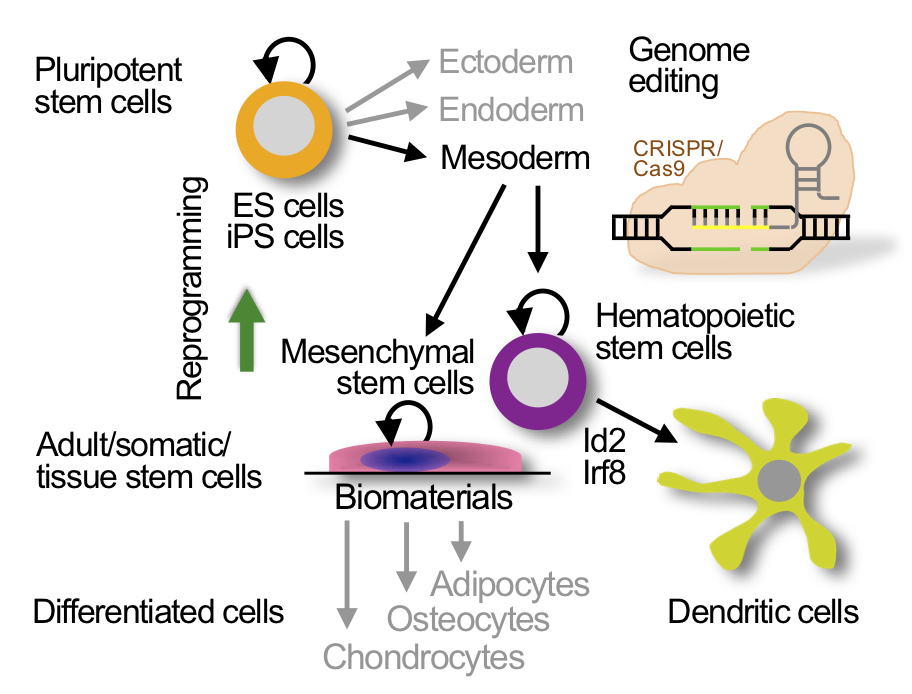 |
The laboratory studies pluripotent stem cells (ES cells and iPS cells) and adult stem cells (hematopoietic stem cells and mesenchymal stem cells). Patient and disease specific iPS cells are obtained from somatic cells by reprogramming. Hematopoietic stem cells are differentiated into dendritic cells. Genome editing with CRISPR/Cas is used for precision genome engineering. |
Gene Expression and Epigenetic Signatures of Hematopoietic Stem Cells
Maintenance of all hematopoietic cells in our organism is achieved by the continuous growth and differentiation of a population of multipotent hematopoietic stem cells. We employ transcriptional profiling with DNA microarrays and RNA-Seq to determine the gene expression repertoire of stem/progenitor cells and their differentiated progeny (Hacker et al., 2003; Hieronymus et al., 2005; Zenke and Hieronymus, 2006; Felker et al., 2010; Lin et al., 2015). Epigenetic signatures of cells are investigated by ChIP-Seq, ATAC-Seq and HiChIP-Seq to elucidate how chromatin architecture determines stemness, differentiation potential and cell identity (Chauvistré et al., 2014; Lin et al., 2015) (see also Epigenetic and Transcriptional Architecture of Dendritic Cell Development).
Stem Cell Engineering
Induced pluripotent stem cells (iPS cells) represent engineered stem cells, which are obtained by reprogramming of somatic cells with specific sets of transcription factors, such as Oct4, Sox2, Klf4 and c-Myc. We found that Ezh2, a core subunit of Polycomb repressive complex 2 (PRC2), is critical for efficient iPS cell generation (Ding et al., 2014). Ezh2 acts during reprogramming at least in part through repressing the Ink4a/Arf locus, which represents a major roadblock for iPS cell generation.
iPS cells provide unique opportunities for disease modeling, drug development and cell therapy. However, frequently their differentiation potential is rather poor. We used cell fusion of iPS cells with hematopoietic stem cells to increase the propensity and differentiation potential of pluripotent stem cells towards hematopoietic cells and other mesendodermal lineages, such as cardiomyocytes, hepatocytes and endothelial cells (Qin et al., 2014).
Immunogenicity of iPS cells and iPS cell-derived cells remains controversial. Sertoli cells constitute the structural framework in testis and provide an immune-privileged environment for germ cells. We found that early-passage Sertoli cell-derived iPS cells retain some somatic memory of Sertoli cells that confers reduced immunogenicity of iPS cells and iPS cell-derived cells in vivo and in vitro (Wang et al., 2014). Our data suggest that immune-privileged Sertoli cells represent a preferred source for iPS cell generation if it comes to the use of iPS cell-derived cells for transplantation.
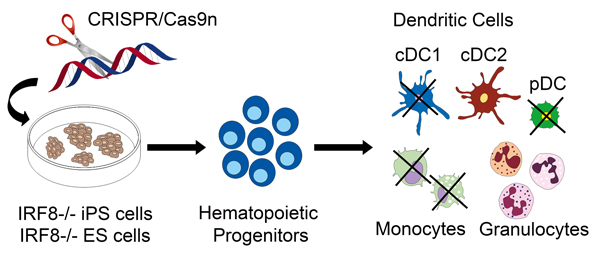 |
Human IRF8-/- iPS cells and IRF8-/- ES cells are obtained by CRISPR/Cas9 genome editing and differentiated into hematopoietic progenitors and their progeny (cDC1 and cDC2, classical dendritic cells type 1 and 2, respectively; pDC, plasmacytoid dendritic cells). |
iPS cells and precision genome engineering with CRISPR/Cas are particularly well suited for cell engineering. We used CRISPR/Cas to generated human iPS cells deficient in IRF8 (interferon regulatory factor 8). IRF8 is a lineage determining transcription factor in hematopoiesis and IRF8-/- iPS cell-derived hematopoietic cells are deficient in development of specific DC subsets and in DC function (Sontag et al., 2017a, 2017b). IRF8-/- iPS cells now allow studying human immune deficiency in vitro, including the pathophysiology of IRF8 deficient DC.
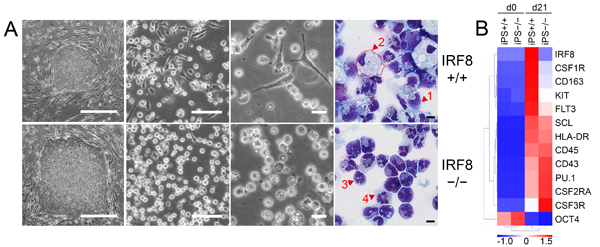 |
(A) Human IRF8-/- and IRF8+/+ iPS cells (left) are induced to differentiate into hematopoietic progenitors and their differentiated progeny (right). (B) Gene expression in IRF8-/- and IRF8+/+ hematopoietic cells depicted in heat map format (red, high and blue, low gene expression) |
Patient- and Disease-specific iPS Cells for Drug Discovery
Patient-specific iPS cells allow modeling aspects of human disease in vitro (Lampert et al., 2020; Toledo et al., 2021). We generated KIT D816V iPS cells from patients with aggressive systemic mastocytosis and mast cell leukemia to develop a patient-specific disease model for mechanistic and drug discovery studies (Toledeo et al., 2021). KIT D816V iPS cells differentiated into neoplastic hematopoietic progenitor cells and mast cells with patient-specific features, thereby reflecting the heterogeneity of the disease. CRISPR/Cas9n-engineered KIT D816V human embryonic stem cells (ES cells), when differentiated into hematopoietic cells, recapitulated the phenotype observed for KIT D816V iPS cell hematopoiesis.
Compound screening identified nintedanib, an FDA approved angiokinase inhibitor that targets VEGFR, PDGFR and FGFR, as a novel KIT D816V inhibitor (Toledeo et al., 2021). Nintedanib selectively reduced the viability of iPS cell-derived KIT D816V hematopoietic progenitor cells and mast cells. Nintedanib was also active on primary samples of KIT D816V patients. Our results suggest nintedanib as a new drug candidate for KIT D816V targeted therapy of advanced systemic mastocytosis and mast cell leukemia.
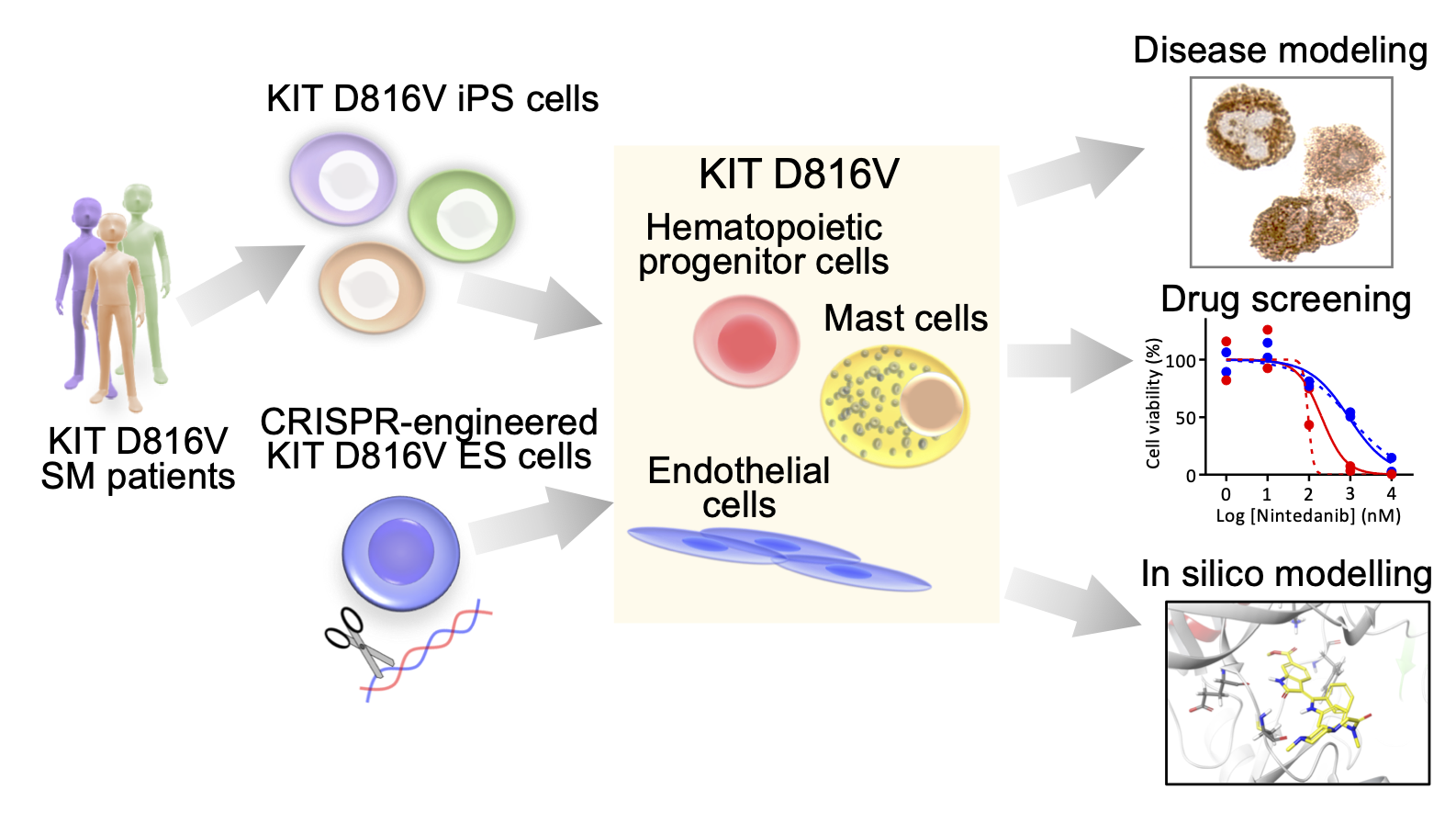 |
KIT D816V iPS cells were obtained by reprogramming blood cells from systemic mastocytosis (SM) patients. The KIT D816V mutation was introduced into human ES cells. KIT D816V iPS cells and ES cells were differentiated into KIT D816V hematopoietic progenitors, mast cells and endothelial cells and used for disease modeling, drug screening and in silico modeling studies (Toledeo et al., 2021). |
To meet the need of obtaining large numbers of iPS cells for disease modeling and drug development, a consortium of experts in engineering sciences and stem cell biology develops an automatic production system for iPS cells, referred to as StemCellFactory (www.stemcellfactory.net, www.stemcellfactory.org).
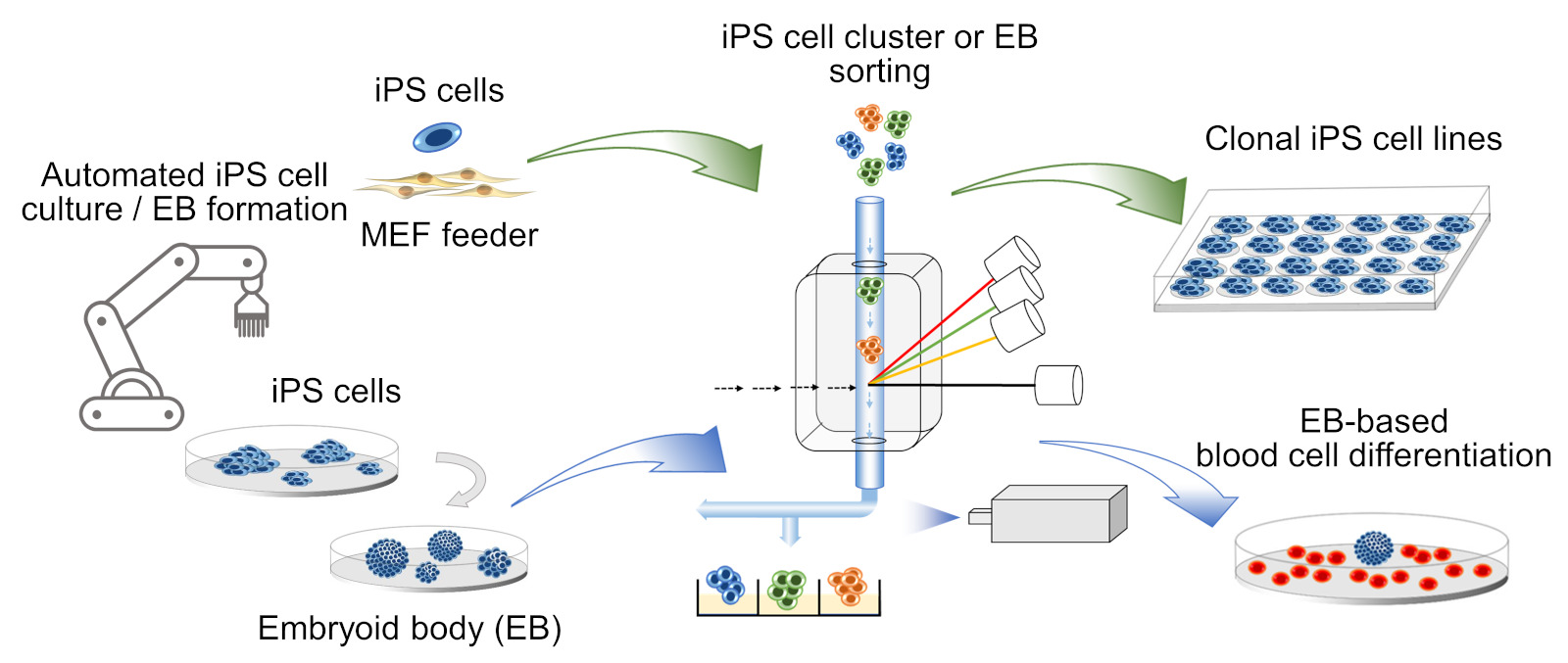 |
Automated iPS cell culture on mouse embryo fibroblast (MEF) feeder and iPS cell cloning with cell cluster sorter for derivation of clonal iPS cell lines (top). Automated embryoid body (EB) formation and EB sorting with cell cluster sorter for blood cell differentiation (bottom) (Ma, Toledo et al., 2022). |
The institute operates a Stem Cell Engineering Unit as a member of the German Stem Cell Cores (German Stem Cell Cores).
RWTH Aachen University and RWTH Aachen University Hospital are members of the Stem Cell Network.NRW.
For human iPS cells of the institute see https://hpscreg.eu
 |
 |
StemCellFactory III: Dieses Vorhaben wurde aus Mitteln des Europäischen Fonds für regionale Entwicklung (EFRE) gefördert |
Selected Publications
| Hijack the HiJAKer: Rethinking Therapy of JAK2-mutant MPN. Zenke, M. and Koschmieder, S. (2025). |
||
| Blood, in press. | ||
| Proinflammatory phenotype of iPS cell-derived JAK2 V617F megakaryocytes induces fibrosis in 3D in vitro bone marrow niche. Flosdorf, N., Böhnke, J., Toledo, M. A. S., Lutterbach, N., Gómez Lerma, V., Grashoff, M., Olschok, K., Gupta, S., Tharmapalan, V., Schmitz, S., Götz, K., Schüler, H. M., Maurer, A., Sontag, S., Küstermann C., Seré, K., Wagner, W., G. Costa, I. G., Brümmendorf, T. H., Koschmieder, S., Chatain, N., Castilho, M., Schneider, R. K. and Zenke, M. (2024). |
||
| Stem Cell Reports 19, 224-238. | Abstract | Full | |
| KIT D816V mast cells derived from induced pluripotent stem cells recapitulate systemic mastocytosis transcriptional profile. Toledo, M. A. S., Fu, X., Maié, T., Buhl, E. M., Götz, K., Schmitz, S., Kaiser, A.,Boor, P., Braunschweig, T., Chatain, N., Costa, I. G., Brümmendorf, T. H., Koschmieder, S. and Zenke, M. (2023). |
||
| Int. J. Mol. Sci. 24, 5275. | Abstract | Full | |
| Towards personalized medicine with iPS cell technology: A case report of advanced systemic mastocytosis with associated eosinophilia. Atakhanov, S., Christen, D., Rolles, B., Schüler, H. M., Panse, J., Chatain, N., Koschmieder, S., Brümmendorf, T. H., Toledo, M. A. S., and Zenke, M. (2022). |
||
| Ann. Hematol., 101, 2533–2536. | Abstract | Full | |
| Dendritic cells generated from induced pluripotent stem cells and by direct reprogramming of somatic cells. Flosdorf, N., and Zenke, M. (2022). |
||
| Eur. J. Immunol. 52, 1880-1888. | Abstract | Full | |
| Cell cluster sorting in automated differentiation of patient-specific induced pluripotent stem cells towards blood cells. Ma, Z., Toledo, M. A. S., Wanek, P., Mabrouk, M. H. E., Smet, F Pulak, R., Pieske, S., Piotrowski, T., Herfs, W., Brecher, C., Schmitt, R. H., Wagner, W., and Zenke, M. (2022). |
||
| Front. Bioeng. Biotech. 10, 755983. | ||
| CRISPR/Cas9-engineered human ES cells harboring heterozygous and homozygous c-KIT knockout. Toledo, M. A. S., Fu, X., Kluge, F., Götz, K., Schmitz, S., Wanek, P., Schüler, H. M., Pannen, K., Chatain, N., Koschmieder, S., Brümmendorf, T. H., and Zenke, M. (2022). |
||
| Stem Cell Research 60, 102732. | ||
| CALR frameshift mutations accelerate maturation of megakaryocytes in MPN patient-derived iPS cells. Olschok, K., Han, L., Toledo, M. A. S., Boehnke, J., Graßhoff, M., Costa, I. G., Theocharides, A., Maurer, A., Schüler, H. M., Buhl, E. M., Pannen, K., Baumeister, J., Kalmer, M., Gupta, S., Boor, P., Gezer, D., Brümmendorf, T. H., Zenke, M., Chatain, N., and Koschmieder, S. (2021). |
||
| Stem Cell Reports 16, 2768-2783. | ||
| CRISPR/Cas9 mediated CXCL4 knockout in human iPS cells of polycythemia vera patient with JAK2 V617F mutation. Boehnke, J., Atakhanov, S., Toledo, M. A. S., Schüler, H. M., Sontag, S., Chatain, N., Koschmieder, S., Brümmendorf, T. H., Kramann, R., and Zenke, M. (2021). |
||
| Stem Cell Research 55, 102490. | ||
| Nintedanib targets KIT D816V neoplastic cells derived from induced pluripotent stem cells of systemic mastocytosis. Toledo, M. A. S., Gatz, M., Sontag, S., Gleixner, K. V., Eisenwort, G., Feldberg, K., Hamouda, A. E. I., Kluge, F., Guareschi, R., Rossetti, G., Sechi, A. S., Dufva, O. M. J., Mustjoki, S. M., Maurer, A., Schüler, H. M., Goetzke, R., Braunschweig, T., Simonowski, A., Panse, J., Jawhar, M., Reiter, A., Hilberg, F., Ettmayer, P., Wagner, W., Koschmieder, S., Brümmendorf, T. H., Valent, P., Chatain, N., and Zenke, M. (2021). |
||
| Blood 137, 2070-2084. | ||
| (see also Commentary by A. Dorrance, Blood 137, 1993-1994, 2021). | ||
| Human sensory neurons derived from pluripotent stem cells for disease modelling and personalized medicine. Lampert, A., Bennett, D. L., McDermott, L. A., Neureiter, A., Eberhardt, E., Winner, B., and Zenke, M. (2020). |
||
| Neurobiol. Pain 8, 100055. | ||
| The StemCellFactory: a modular system integration for automated generation and expansion of human induced pluripotent stem cells. Elanzew, A., Nießing, B., Langendoerfer, D., Rippel, O., Piotrowski, T., Schenk, F., Kulik, M., Peitz, M., Breitkreuz, Y., Jung, S., Wanek, P., Stappert, L., Schmitt, R. H., Haupt, S., Zenke, M., König, N., and Brüstle, O. (2020). |
||
| Front. Bioeng. Biotech. 8, 580352. |
| Full
|
|
| The role of Nav1.7 in human nociceptors: insights from human iPS cell-derived sensory neurons of erythromelalgia patients. Meents, J. E., Bressan, E., Sontag, S., Foerster, A., Hautvast, P., Rösseler, C., Hampl, M., Schüler, H., Goetzke, R., Chi Le, T. K., Kleggetveit, I. P., Le Cann, K., Kerth, C., Rush, A. M., Rogers, M., Kohl, Z., Schmelz, M., Wagner, W., Jørum, E., Namer, B., Winner, B., Zenke, M., and Lampert, A. (2019). |
||
| Pain 160, 1327-1341. | ||
| Differentiation of human induced pluripotent stem cells (iPS cells) and embryonic stem cells (ES cells) into dendritic cell (DC) subsets. Sontag, S., Förster, M., Seré, K. and Zenke, M. (2017b). |
||
| Bio-protocol 7, e2419. | ||
| Modelling IRF8 deficient human hematopoiesis and dendritic cell development with engineered induced pluripotent stem cells. Sontag, S., Förster, M., Qin, J., Wanek, P., Mitzka, S., Schüler, H. M., Koschmieder, S., Rose-John, S., Seré, K. and Zenke, M. (2017a). |
||
| Stem Cells 35, 898-908. | ||
| Reduced immunogenicity of induced pluripotent stem cells derived from Sertoli cells. Wang, X., Qin, J., Zhao, R.C., and Zenke. M. (2014). |
||
| PLoS One 9, e106110. | ||
| Cell fusion enhances mesendodermal differentiation of human induced pluripotent stem cells. Qin, J., Sontag, S., Lin, Q., Mitzka, S., Leisten, I., Schneider, R.K., Wang, X., Jauch, A., Peitz, M., Brüstle, O., Wagner, W., Zhao, R.C., and Zenke, M. (2014). |
||
| Stem Cells Dev. 23, 2875-2882. | ||
| The polycomb protein Ezh2 impacts on induced pluripotent stem cell generation. Ding, X., Wang, X., Sontag, S., Qin, J., Wanek, P., Lin, Q., and Zenke, M. (2014). |
||
| Stem Cells Dev. 23, 931-940. | ||
| Polycomb group protein Bmi1 promotes hematopoietic cell development from ES cells. Ding, X., Lin, Q., Ensenat-Waser, R., Rose-John, S., and Zenke, M. (2012). |
||
| Stem Cells Dev. 21, 121-132. | ||
| Human adult germline stem cells in question. Ko, K., Araúzo-Bravo, M. J., Tapia, N., Kim, J., Lin, Q., Bernemann, C., Han, D. W., Gentile, L., Reinhardt, P., Greber, B., Schneider, R. K., Kliesch, S., Zenke, M., and Schöler, H. R. (2010). |
||
| Nature 465, E1-E3. | ||
| Pluripotent stem cells induced from adult neural stem cells by reprogramming with two factors. Kim, J. B., Zaehres, H., Wu, G., Gentile, L., Ko, K., Sebastiano, V., Arauzo-Bravo J. M., Ruau, D., Han, D. W., Zenke, M., and Schöler H. R. (2008). |
||
| Nature, 454, 646-650. | ||
| Pluripotency associated genes are reactivated by chromatin modifying agents in neurosphere cells. Ruau, D., Ensenat-Waser, R., Dinger, T. C., Vallabhapurapu, D. S., Rolletschek, A., Hacker, C., Hieronymus, T., Wobus, A. M., Müller, A. M., and Zenke, M. (2008). |
||
| Stem Cells 26, 920-926. | ||